
In less acute circumstances, epinephrine can be given as a continuous infusion.
#Somatic nervous system effector organs free
Epinephrine also has endocrine and metabolic effects that include increasing the levels of blood glucose, lactate, and free fatty acids.Īn intravenous dose of 1 mg can be given for cardiovascular collapse, asystole, ventricular fibrillation, pulseless electrical activity, or anaphylactic shock to constrict the peripheral vasculature and maintain myocardial and cerebral perfusion. The effects that predominate depend on the dose of epinephrine administered. Among the therapeutic effects of epinephrine are positive inotropy, chronotropy, and enhanced conduction in the heart (β1) smooth muscle relaxation in the vasculature and bronchial tree (β2) and vasoconstriction (α1). It is also commonly used locally to decrease the systemic absorption of local anesthetics and to reduce surgical blood loss. Exogenous epinephrine is used intravenously in life-threatening circumstances to treat cardiac arrest, circulatory collapse, and anaphylaxis. Like norepinephrine, epinephrine binds to α- and β- adrenergic receptors. Prolonged infusion of norepinephrine can also cause ischemia in the fingers and toes because of the marked peripheral vasoconstriction.
Additionally, because norepinephrine vasoconstricts the pulmonary, renal, and mesenteric circulations, infusions must be carefully monitored to prevent injury to vital organs. The increase in systemic resistance can lead to reflex bradycardia. Like all the endogenous catecholamines, the half-life of norepinephrine is short (2.5 minutes), so it is usually given as a continuous infusion at rates of 3 μg/min or more and titrated to the desired effect. It is used primarily for its α1-adrenergic effects that increase systemic vascular resistance. Norepinephrine, the primary adrenergic neurotransmitter, binds to α- and β-receptors. Norepinephrine that escapes this reuptake process and makes its way into the circulation is metabolized by either the monoamine oxidase (MAO) or catechol- O-methyltransferase (COMT) enzyme in the blood, liver, or kidney. Norepinephrine is then released from these receptors and mostly taken up at the presynaptic nerve terminal and transported to storage vesicles for reuse. The postsynaptic receptors then activate secondary messenger systems in the postsynaptic cell via G protein–linked activity. The released norepinephrine binds to the pre- and postsynaptic adrenergic receptors. In general, only 1% of the total stored norepinephrine is released with each depolarization thus, there is a tremendous functional reserve. Then the vesicles merge with the cell membrane and release their contents into the synapse ( Fig.5). The neurotransmitters are stored in vesicles until the postganglionic nerve is stimulated. In the adrenal medulla, norepinephrine is methylated to epinephrine. DOPA is then converted to dopamine and, once inside the storage vesicle at the nerve terminal, is β-hydroxylated to norepinephrine. The rate-limiting step is the transformation of tyrosine to dihydroxyphenylalanine (DOPA), which is catalyzed by the enzyme tyrosine hydroxylase. Sympathetic neurotransmitters are synthesized from tyrosine in the postganglionic sympathetic nerve ending ( Fig.4).
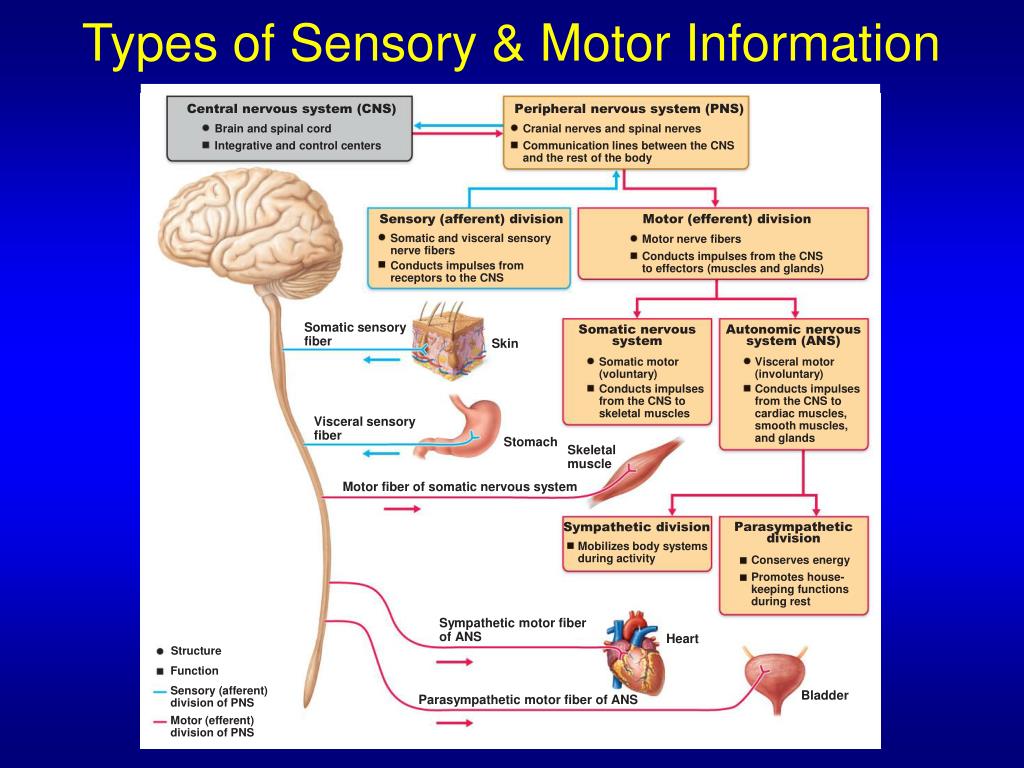
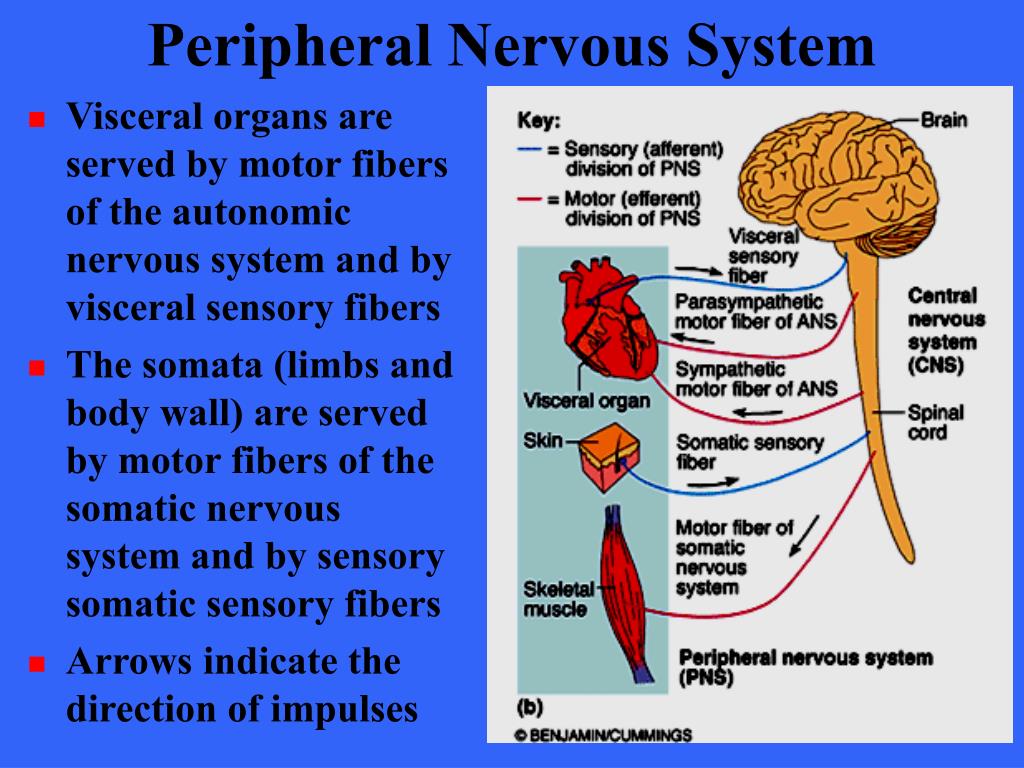
In contrast, the postganglionic fibers run a long course before innervating effector organs ( Fig.2). The sympathetic preganglionic fibers are relatively short because sympathetic ganglia are generally close to the central nervous system (CNS). The postganglionic neurons of the SNS then travel to the target organ. A sympathetic response, therefore, is not confined to the segment from which the stimulus originates, as discharge can be amplified and diffuse. Preganglionic sympathetic fibers not only synapse at the ganglion of the level of their origin in the spinal cord but can also course up and down the paired ganglia.
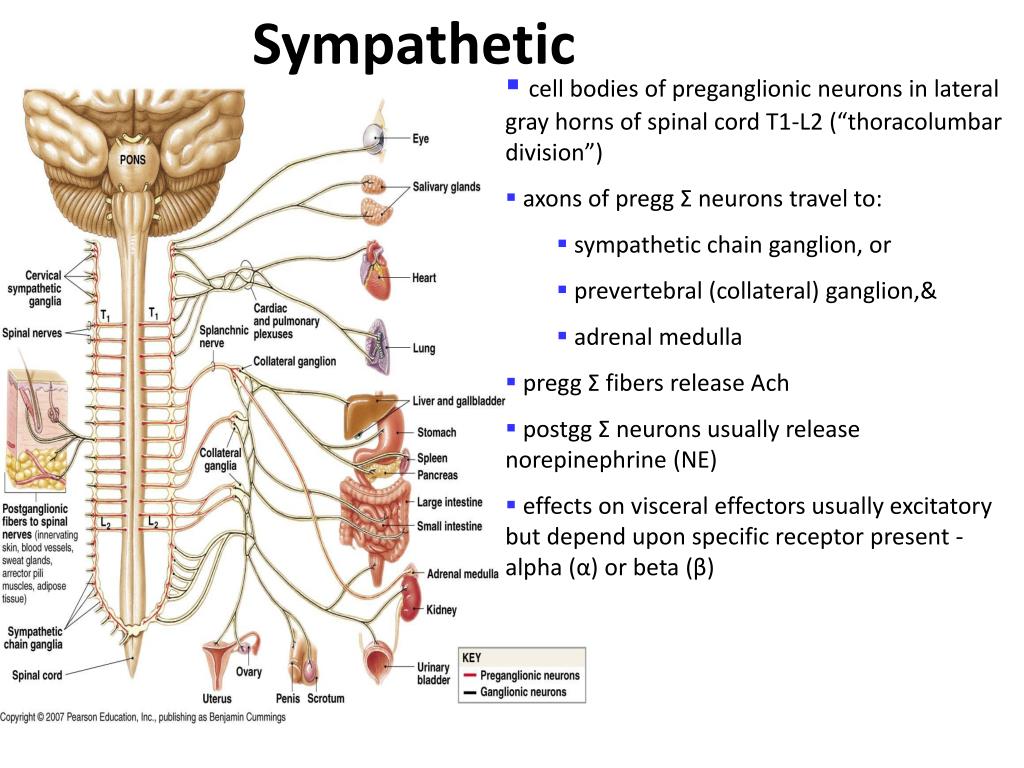
The nerve fibers extend to paired ganglia, creating the sympathetic chains that lie immediately lateral to the vertebral column or extend to unpaired distal plexuses (e.g., the celiac and mesenteric plexuses). The cell bodies of these neurons lie in the spinal gray matter. The preganglionic fibers of the SNS originate from the thoracolumbar region (T1 to L2 or 元) of the spinal cord ( Fig.1).


 0 kommentar(er)
0 kommentar(er)
